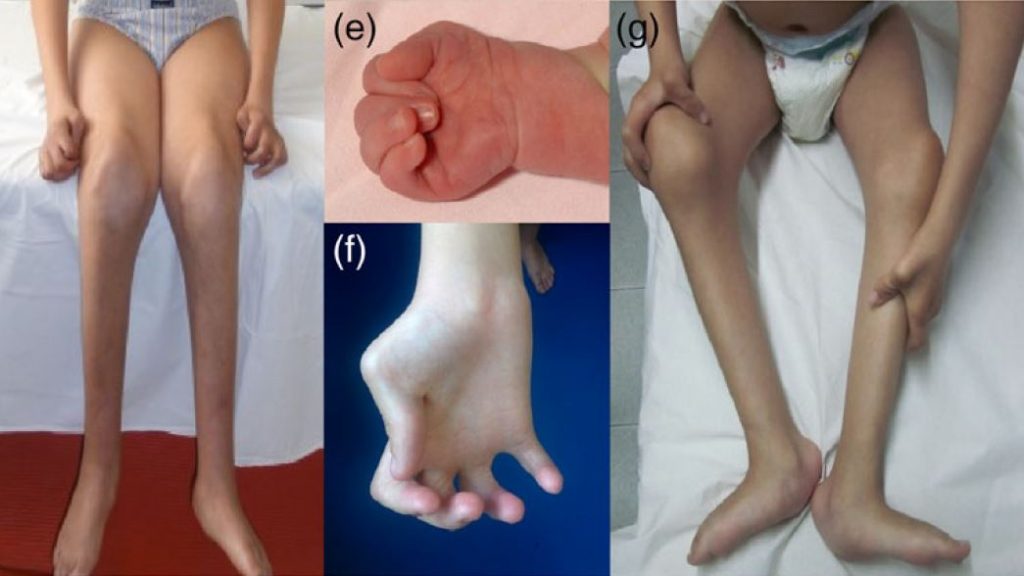
New publication: CHRNG-related nonlethal multiple pterygium syndrome: Muscle imaging pattern and clinical, histopathological, and molecular genetic findings
A group of investigators from the Sant Joan de Deu hospital in Barcelona (Carrera, Natera and Nascimento) led a comprehensive study looking at muscle outcomes for patients with defects in the gamma-subunit of the acetylcholine receptor. In humans, the gamma subunit is only expressed before birth and for a short time afterwards. Biallelic loss-of-function mutations are believed to cause neuromuscular transmission failure in utero, resulting in fewer or no fetal movements and multiple joint contractures, or a prenatal myasthenic syndrome. After a certain stage in development, the gamma subunit is no longer required and completely replaced by the so-called adult epsilon subunit, enabling neuromuscular transmission to be restored. Thereafter the clinical course is typically stable. In individuals surviving beyond this point, the disorder is often known as Escobar syndrome or nonlethal multiple pterygium syndrome (MPS) and requires differentiating from other forms of arthrogryposis multiplex congenita. This comprehensive overview looked at the long-term outcomes of the condition, assessing clinical, biopsy and MRI findings as well as molecular genetic features, and identified long-term changes to the muscle that may be of functional relevance for the patients.
The study was published in the American Journal of Medical Genetics and the publication is now available at the journal website.

Communicated by Daniel Natera
Carrera-García L, Natera-de Benito D, Dieterich K, de la Banda MGG, Felter A, Inarejos E et al.
CHRNG-related nonlethal multiple pterygium syndrome: Muscle imaging pattern and clinical, histopathological, and molecular genetic findings.
Am. J. Med. Genet. A. 2019.
PMID: 30868735
DOI: 10.1002/ajmg.a.61122
Abstract
Mutations in the CHRNG gene cause autosomal recessive multiple pterygium syndrome (MPS). Herein we present a long-term follow-up of seven patients with CHRNG-related nonlethal MPS and we compare them with the 57 previously published patients. The objective is defining not only the clinical, histopathological, and molecular genetic characteristics, but also the type and degree of muscle involvement on whole-body magnetic resonance imaging (WBMRI). CHRNG mutations lead to a distinctive phenotype characterized by multiple congenital contractures, pterygium, and facial dysmorphism, with a stable clinical course over the years. Postnatal abnormalities at the neuromuscular junction were observed in the muscle biopsy of these patients. WBMRI showed distinctive features different from other arthrogryposis multiple congenita. A marked muscle bulk reduction is the predominant finding, mostly affecting the spinal erector muscles and gluteus maximus. Fatty infiltration was only observed in deep paravertebral muscles and distal lower limbs. Mutations in CHRNG are mainly located at the extracellular domain of the protein. Our study contributes to further define the phenotypic spectrum of CHRNG-related nonlethal MPS, including muscle imaging features, which may be useful in distinguishing it from other diffuse arthrogryposis entities.