New Publication: A phase 3 randomized study evaluating sialic acid extended-release for GNE myopathy
Our most recent paper reporting on a phase 3 international multi-center study (13 sites across 7 countries) to evaluate extended-release sialic acid as a therapy option for GNE myopathy is now available to read online.
Dr Lochmüller led an international group of investigators in a phase 3 clinical trial sponsored by Ultragenyx Pharmaceutical Inc with full results now published in Neurology. The study showed that a slow-release formula of sialic acid was not superior to placebo in improving muscle strength and function in patients with GNE myopathy (also called hereditary inclusion body myopathy or Nonaka distal myopathy) despite increasing serum sialic acid levels. This is in contrast to previous positive results in a mouse model and in a phase 2 clinical trial. While this result is disappointing for patients and families with GNE myopathy, it is relevant for several reasons: The study is the first multinational phase 3 trial in any distal myopathy and provides important information on trial feasibility, recruitment, outcome measures and natural progression, which will aid planning and execution of future clinical trials in GNE myopathy. The result also opens the question whether GNE myopathy is a sialic acid deficiency syndrome and whether the GNE protein has other yet to be defined functions inside muscle cells. This also demonstrates the importance of publishing so-called negative results.
Read the full paper on the Neurology website here (open access).
Abstract
Objective
To investigate the efficacy and safety of aceneuramic acid extended-release (Ace-ER), a treatment intended to replace deficient sialic acid, in patients with GNE myopathy.
Methods
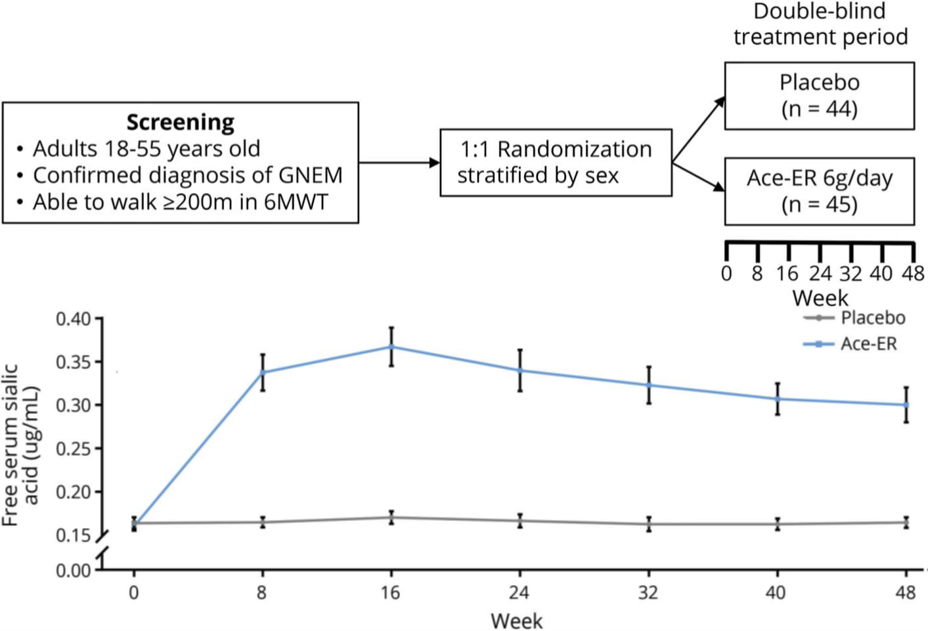
UX001-CL301 was a phase 3, double-blind, placebo-controlled, randomized, international study evaluating the efficacy and safety of Ace-ER in patients with GNE myopathy. Participants who could walk ≥200 meters in a 6-minute walk test at screening were randomized 1:1, and stratified by sex, to receive Ace-ER 6 g/d or placebo for 48 weeks and assessed every 8 weeks.
The primary endpoint was change in muscle strength over 48 weeks measured by upper extremity composite (UEC) score. Key secondary endpoints included change in lower extremity composite (LEC) score, knee extensor strength, and GNE myopathy–Functional Activity Scale (GNEM-FAS) mobility domain score. Safety assessments included adverse events (AEs), vital signs, and clinical laboratory results.
Results
Eighty-nine patients were randomized (Ace-ER n = 45; placebo n = 44). Change from baseline to week 48 for UEC score between treatments did not differ (least square mean [LSM] Ace-ER −2.25 kg vs placebo −2.99 kg; LSM difference confidence interval [CI] 0.74 [−1.61 to 3.09]; p = 0.5387). At week 48, there was no significant difference between treatments for the change in key secondary endpoints: LEC LSM difference (CI) −1.49 (−5.83 to 2.86); knee extension strength −0.40 (−2.38 to 1.58); and GNEM-FAS mobility domain score −0.72 (−2.01 to 0.57).
Gastrointestinal events were the most common AEs.
Conclusions
Ace-ER was not superior to placebo in improving muscle strength and function in patients with GNE myopathy.
Classification of evidence
This study provides Class I evidence that for patients with GNE myopathy, Ace-ER does not improve muscle strength compared to placebo.
Trial Registration
NCT02377921, 2014-005432-33