New publication on shared decision-making puts the patient perspective front and centre
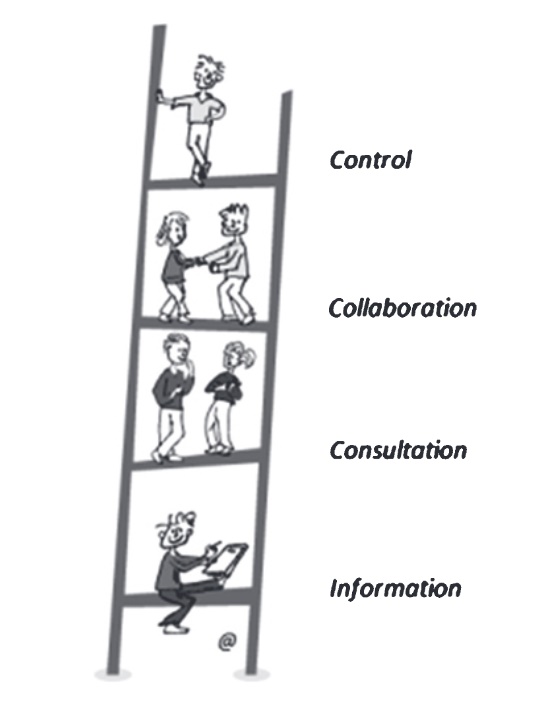
How do we ensure patients are involved in neuromuscular research right from its earliest stages? Shared decision-making (SDM), in which doctors and patients jointly decide on treatment or care, has emerged as a gold standard model of healthcare. Within neuromuscular research, obtaining the patient perspective on matters such as research objectives, study design, or even consent, had received relatively less attention until recently. This new publication presents the results of a 2018 workshop hosted by the European Neuromuscular Centre (ENMC), a group of patient organisations for neuromuscular disorders. The workshop brought together SDM experts with neuromuscular clinicians, researchers, drug developers and patient representatives and resulted in a position paper designed to be a starting point to improve future practice across the neuromuscular research field. Examples of successful patient involvement in patient registries, biobanks and clinical trial participation were presented, and the areas where this could be further extended and improved were discussed. In order to achieve this transformation, educational, structural and cultural changes need to be implemented by all stakeholders: patients and patient organizations, healthcare professionals and regulatory bodies.
The publication is open-access and is available online at the website of the Journal of Neuromuscular Diseases here.
The Position of Neuromuscular Patients in Shared Decision Making: Report from the 235th ENMC Workshop: Milan, Italy, January 19-20, 2018. Hanns Lochmüller, Anna Ambrosini, Baziel van Engelen, Mats Hansson, Aad Tibben, Alexandra Breukel, Ellen Sterrenburg, Guus Schrijvers, Ingeborg Meijer, George Padberg, Holly Peay, Lucia Monaco, Mike Snape, Anne Lennox, Elena Mazzone, Nathalie Bere, Mencia de Lemus, Erik Landfeldt, and Raffaella Willmann on behalf of the 235th ENMC workshop study group. Journal of Neuromuscular Diseases, Volume 6, Issue 1 (February 2019)
DOI 10.3233/JND-180368.
Abstract
In the era of patient-centered medicine, shared decision-making (SDM) – in which healthcare professionals and patients exchange information and preferences and jointly reach a decision – has emerged as the gold standard model for the provision of formal healthcare. Indeed, in many geographical settings, patients are frequently invited to participate in choices concerning the design and delivery of their medical management. From a clinical perspective, benefits of this type of patient involvement encompass, for example, enhanced treatment satisfaction, improved medical compliance, better health outcomes, and maintained or promoted quality of life. Yet, although the theory and enactment of SDM in healthcare are well-described in the literature, comparatively less attention has been devoted to contextualizing questions relating to if, when, and how to include patients in decisions within medical research. In this context, patient involvement would be expected to be potentially relevant for and applicable to a wide range of activities and processes, from the identification of research priorities and development of grant applications, to the design of patient information and consent procedures, formulation of interventions, identification and recruitment of study sample populations, feasibility of a clinical trial, identification, selection, and specification of endpoints and outcomes in clinical trials and observational studies, data collection and analysis, and dissemination of results. To this end, 45 clinicians, healthcare professionals, researchers, patients, caregivers, and representatives from regulatory authorities and pharmaceutical companies from 15 different countries met to discuss the level of involvement of patients with neuromuscular diseases, specifically in the following settings of medical research for neuromuscular diseases: i) registries and biobanks; ii) clinical trials; and iii) regulatory processes. In this report, we present summaries of the talks that were given during the workshop, as well as discussion outcomes from the three topic areas listed above.