New publication: Targeted therapies for congenital myasthenic syndromes: systematic review and steps towards a treatabolome
Our new publication by Thompson et al on the treatments available for the congenital myasthenic syndromes (CMS) and the development of a “treatabolome” to improve the accessibility of information on treatable rare diseases is now available online.
In this publication we look into ways we can improve access to the important information that a particular disease has a treatment available. Most rare genetic diseases do not currently have curative gene-based therapies available, but in some cases a particular drug or intervention can have a significant effect on disease course and functional ability. Ideally, this information would be immediately accessible to the clinician treating the patient at the time of diagnosis, but too often this is not the case and the details can only be discovered after more extensive literature study or expert referral, meaning that for many patients there is a delay before they are put on the optimal therapy. The question is further complicated by the fact that some treatments are only effective for certain genetic causes, even when the overall phenotypic presentation is very similar.
We use the example of CMS, rare but highly treatable neuromuscular diseases, to explore this question. We performed a systematic review of the literature to extract treatment evidence for each CMS subtype linked to genetic details and conclude that even though there is very little evidence from randomised controlled trials, which would be considered the top level of evidence to validate treatment effect, all known CMS subtypes do in fact receive treatment in clinical practice and this information is worthy of presenting to the clinician in a more easily accessible fashion. We propose that just as information about the pathogenicity of specific variants is captured in online databases thanks to major curation efforts by the international diagnostic community, similar open databases curated by disease experts could capture evidence on treatment options linked to variant details in a so-called “treatabolome”, in order that this information could be flagged up to the clinician at the time of diagnosis. The decision about how to treat a patient is still a matter for expert clinical judgement and cannot be left to a computer algorithm, but what the computer algorithm can do is help ensure that the clinician has the evidence available to inform their judgement. This approach is currently being trialled by the European Solve-RD project, where disease experts from several different European Reference Networks will perform similar reviews of the evidence and bioinformaticians from the Centro Nacional de Análisis Genómico (CNAG-CRG) in Barcelona will make them accessible in database form for incorporation into genomic analysis platforms such as RD-Connect.
The full publication can be read at the Emerging Topics in Life Sciences website here.
Targeted therapies for congenital myasthenic syndromes: systematic review and steps towards a treatabolome
Rachel Thompson, Gisèle Bonne, Paolo Missier and Hanns Lochmüller
Emerging Topics in Life Sciences (2019)
https://doi.org/10.1042/ETLS20180100
Abstract
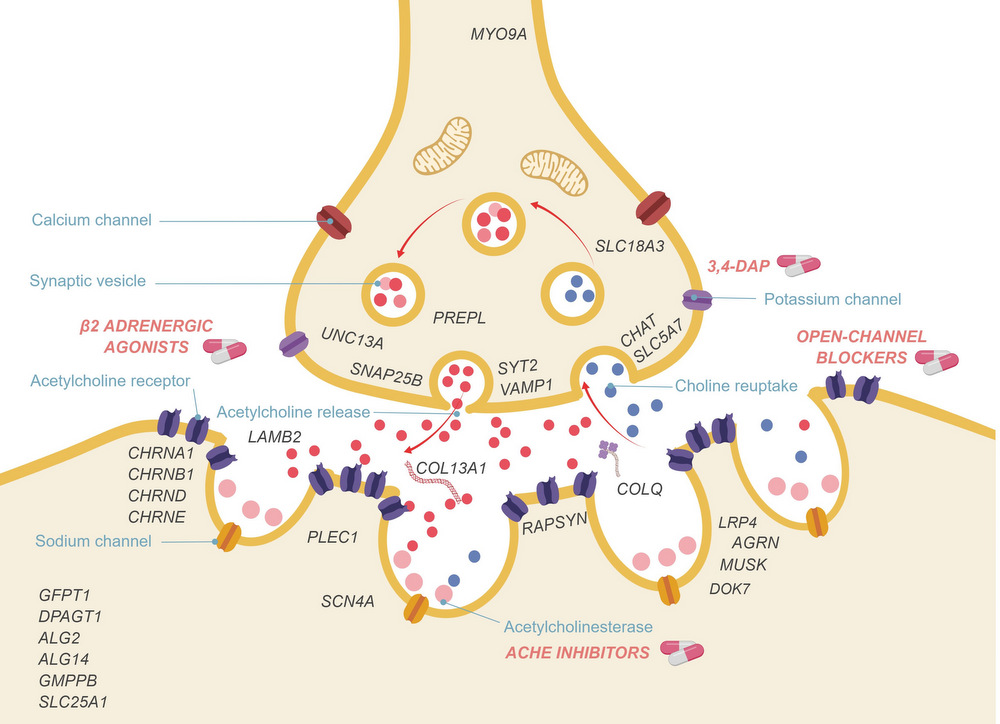
Despite recent scientific advances, most rare genetic diseases — including most neuromuscular diseases — do not currently have curative gene-based therapies available. However, in some cases, such as vitamin, cofactor or enzyme deficiencies, channelopathies and disorders of the neuromuscular junction, a confirmed genetic diagnosis provides guidance on treatment, with drugs available that may significantly alter the disease course, improve functional ability and extend life expectancy. Nevertheless, many treatable patients remain undiagnosed or do not receive treatment even after genetic diagnosis. The growth of computer-aided genetic analysis systems that enable clinicians to diagnose their undiagnosed patients has not yet been matched by genetics-based decision-support systems for treatment guidance. Generating a ‘treatabolome’ of treatable variants and the evidence for the treatment has the potential to increase treatment rates for treatable conditions. Here, we use the congenital myasthenic syndromes (CMS), a group of clinically and genetically heterogeneous but frequently treatable neuromuscular conditions, to illustrate the steps in the creation of a treatabolome for rare inherited diseases. We perform a systematic review of the evidence for pharmacological treatment of each CMS type, gathering evidence from 207 studies of over 1000 patients and stratifying by genetic defect, as treatment varies depending on the underlying cause. We assess the strength and quality of the evidence and create a dataset that provides the foundation for a computer-aided system to enable clinicians to gain easier access to information about treatable variants and the evidence they need to consider.
Summary points
- A significant number of rare diseases are treatable with marketed therapies or commonly available drugs, but ensuring that patients receive the appropriate treatment in a timely manner remains challenging and requires new technologies suitable for the genomic era to support clinicians in accessing the relevant information.
- Very little high-level evidence exists for the drug treatment of congenital myasthenic syndromes (CMS), but an increasing body of expert opinion based on clinical evidence suggests that highly efficacious treatments do exist for the majority of patients and that treatment must be tailored to the underlying genetic defect. Here, we link genotype with treatment information for all CMS subtypes based on a systematic review of over 200 publications on more than 1000 patients.
- While expert opinion and systematic reviews may provide guidance and better access to evidence, finding the best treatment for an individual patient involves complex decision processes that remain the responsibility of an experienced clinician. Further prospective and controlled studies of treated CMS patients are required to provide better evidence for the long-term safety and efficacy of medications commonly used off-label.
- Next-generation sequencing is increasingly used as a first-line diagnostic tool for patients with unspecific neuromuscular complaints or weakness, some of whom may be affected by CMS. To alert the treating geneticist or clinician about patients with this highly treatable condition at the time of reviewing NGS results, we will integrate the information from this systematic review into a computer-readable and interoperable knowledgebase, the treatabolome, and expand the concept to other rare diseases with the buy-in of domain experts.