Myotonic dystrophy
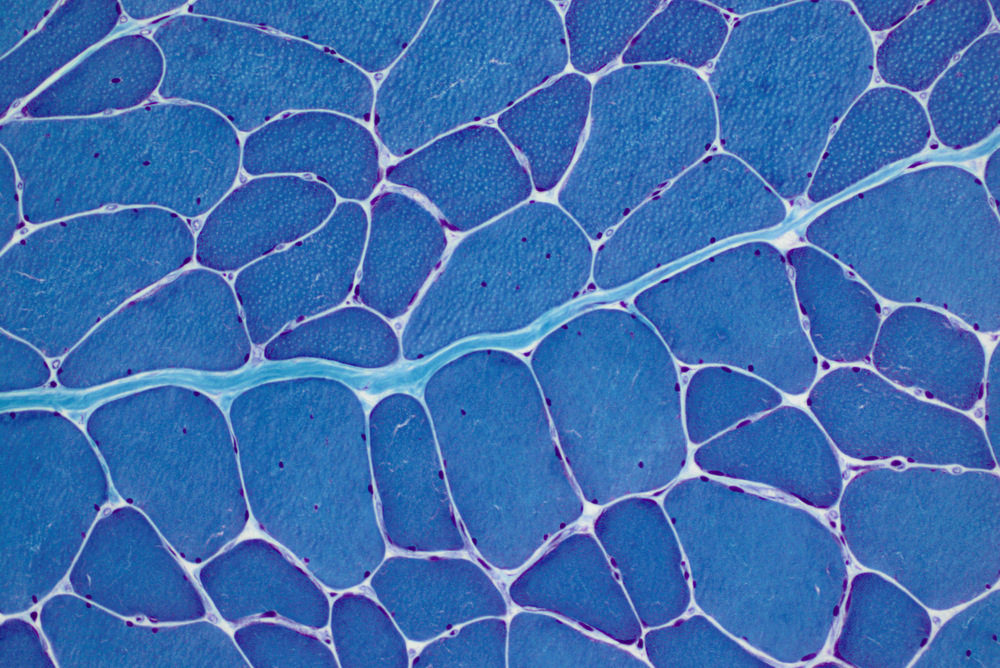
Myotonic dystrophy type 1 (DM1) is the most common form of muscular dystrophy in adults. It is a complex, multi-systemic and progressive disorder that leads to severe fatigue, substantial physical functional impairment, and restricted social participation. DM1 is caused by a triplet repeat expansion in the DMPK gene and has autosomal dominant inheritance. There is currently no licensed therapy to reverse, slow down or cure the symptoms of this condition, but specific symptoms may be improved by some drugs or other interventions.
Read more about myotonic dystrophy at the NIH Genetics Home Reference site here.
Our group is interested in translational research in all areas of this complex disease. We have significant experience in both clinical and basic research projects in this area and are keen to forge collaborations that improve trial readiness. In Canada, we see a large population of patients with DM1 and work with Canadian colleagues and the Canadian Neuromuscular Disease Registry to involve them in clinical research.
Clinical Research
Hanns is an internationally recognized opinion leader in this area and has been instrumental in carrying out the first clinical trials for DM1 in Europe. Notably, he was one of the clinical leads in the UK, together with Dr Grainne Gorman, for the EU-funded trial OPTIMISTIC. This was the first international multi-centre clinical trial with intervention in DM1. Led by Prof Baziel van Engelen from Nijmegen, the study also included sites in Paris (led by Guillaume Bassez) and Munich (led by Benedikt Schoser). It explored whether cognitive behavioural therapy and graded exercise would be beneficial to the quality of life of these patients. Importantly, as well as answering the specific research question and revealing that these interventions do benefit patients (Okkersen, K, Lancet Neurol. 2018), the study also showed that a large-scale study (over 200 patients) in this population is possible, which paves the way to further trials.
In addition, Hanns led the largest longitudinal natural history study in myotonic dystrophy in the UK. This now continues under the leadership of Dr Chris Turner (UCLH), Dr Nikoletta Nikolenko and Dr Grainne Gorman (Newcastle).
Hanns has also taken a leading role in engaging with pharmaceutical companies to increase interest in myotonic dystrophy type 1 and was instrumental starting the first commercially funded trial in Europe.
Patient Registries
Hanns initiated the development of a minimal core dataset for DM1 patient registries (Thompson et al Neuromuscul Disord. 2009) and established the UK Patient Registry for myotonic dystrophy together with Muscular Dystrophy UK and the Myotonic Dystrophy Support Group in 2011. He led the registry to over 500 participants over 6 years (Wood et al J Neurol. 2017). The registry supported many research studies and saw the research being carried out in this area in the UK increase significantly. It supported a range of research projects including studies funded by the National Institutes of Health (US)(Best et al Eur. J. Neurol. 2018 ) and National Institute of Health Research (UK).
In Canada, our group works closely with the Canadian Neuromuscular Disease Registry to register patients and develop cohorts for clinical research. We also actively foster international collaborations to accelerate research in this population, as was highlighted in a recent review into the current status of DM1 registries globally (Wood et al OJRD 2018).
Biomarkers
Our group has worked with collaborators to better identify biomarkers that can be used to understand disease progression which can ultimately be validated and be used as surrogate outcome measures in therapy development. This work has covered a broad range of areas including:
- MRI (cardiac and skeletal)
- Proteomics
- RNASeq