New Publication: Bi-allelic variants of FILIP1 cause congenital myopathy, dysmorphism and neurological defects
Lochmüller Lab study on novel disease gene published in BRAIN: a journal of neurology.
Filamin-A-interacting protein 1 (FILIP1) is a structural protein that is involved in neuronal and muscle function and integrity. Our new paper shows that loss of functional FILIP1 causes a recessive disorder characterized by neurological and muscular manifestations as well as dysmorphic features accompanied by perturbed proteostasis and myopathology. While the filamin-encoding genes FLNA and FLNC have previously been linked to neurological and muscle disorders, this is the first time FILIP1 has been associated with human disease.
Bi-allelic variants of FILIP1 cause congenital myopathy, dysmorphism and neurological defects is now available in BRAIN: a journal of neurology. The publication is authored by associated scientist Andreas Roos together with Kaela O’Connor and Hanns Lochmüller.
The publication reports on five patients from four unrelated families presenting with neurological and muscular symptoms who have bi-allelic variants in FILIP1. With these case reports and several characterization studies on the investigated variants, FILIP1 was identified as a novel genetic cause for a cluster of congenital neuropediatric symptoms. This identification provides a genetic diagnosis for the five patients involved in this research, and highlights FILIP1 as a potential cause for undiagnosed patients with similar symptoms.
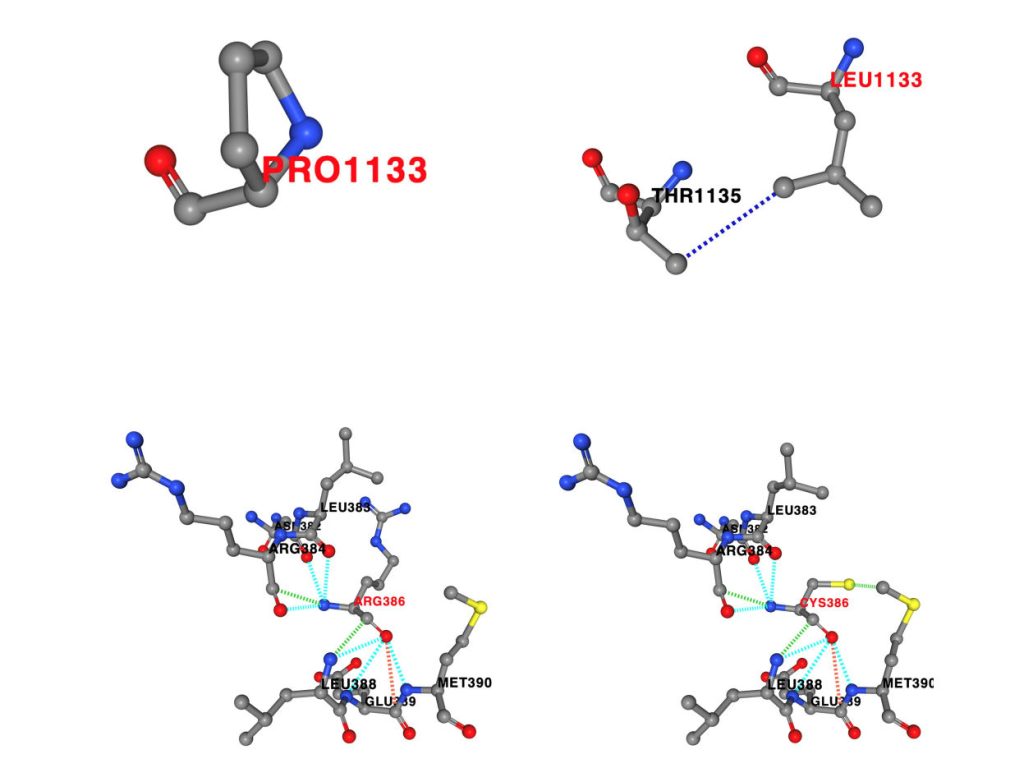
Our lab performed in silico studies on the identified missense variants to predict alterations to the non-covalent interactions within the protein structure, and to predict changes to protein stability.
How to Find the Publication
This paper is not open access; If you do not have access through your institution, please contact Dr Hanns Lochmüller to obtain a full-text version.
Reference
Roos, A., van der Ven, P. F. M., Alrohaif, H., Kölbel, H., Heil, L., Della Marina, A., Weis, J., Aßent, M., Beck-Wödl, S., Barresi, R., Töpf, A., O’Connor, K., Sickmann, A., Kohlschmidt, N., El Gizouli, M., Meyer, N., Daya, N., Grande, V., Bois, K., Kaiser, F. J., … Lochmüller, H. (2023). Bi-allelic variants of FILIP1 cause congenital myopathy, dysmorphism and neurological defects. Brain : a journal of neurology, awad152. Advance online publication. https://doi.org/10.1093/brain/awad152
Abstract
Filamin-A-interacting protein 1 (FILIP1) is a structural protein that is involved in neuronal and muscle function and integrity and interacts with FLNa and FLNc. Pathogenic variants in filamin-encoding genes have been linked to neurological disorders (FLNA) and muscle diseases characterized by myofibrillar perturbations (FLNC), but human diseases associated with FILIP1 variants have not yet been described. Here, we report on five patients from four unrelated consanguineous families with homozygous FILIP1 variants (two nonsense and two missense). Functional studies indicated altered stability of the FILIP1 protein carrying the p.[Pro1133Leu] variant. Patients exhibit a broad spectrum of neurological symptoms including brain malformations, neurodevelopmental delay, muscle weakness and pathology, and dysmorphic features. Electron and immunofluorescence microscopy on the muscle biopsy derived from the patient harbouring the homozygous p.[Pro1133Leu] missense variant revealed core-like zones of myofibrillar disintegration, autophagic vacuoles and accumulation of FLNc. Proteomic studies on the fibroblasts derived from the same patient showed dysregulation of a variety of proteins including FLNc and alpha-B-crystallin, a finding (confirmed by immunofluorescence) which is in line with the manifestation of symptoms associated with the syndromic phenotype of FILIP1opathy. The combined findings of this study show that the loss of functional FILIP1 leads to a recessive disorder characterized by neurological and muscular manifestations as well as dysmorphic features accompanied by perturbed proteostasis and myopathology.