New Publication: Clinical and genetic characterisation of a large Indian congenital myasthenic syndrome cohort
Lochmüller Lab study identifying and genetically characterising the largest-ever Indian cohort of congenital myasthenic syndrome patients published in BRAIN: a journal of neurology.
Congenital myasthenic syndromes (CMS) are a rare group of inherited disorders caused by defects in genes that are associated with the neuromuscular junction. Unlike many neuromuscular conditions, CMS is often treatable with commonly available medications such as acetylcholinesterase inhibitors and beta2 adrenergic receptor agonists. To select the correct treatment, it is essential to identify the genetic cause of the patient’s disease. CMS is extremely rare and over 35 genes causing the condition have been identified to date. There is still insufficient knowledge about geographic variability in genetic subtypes of CMS and the full genetic and phenotypic spectrum of the disease. Characterising large cohorts of patients is crucial to developing an understanding of how the broad genetic spectrum of the disease presents symptomatically and identifying novel CMS-causing genes. This will reduce the time it takes for patients to receive a CMS diagnosis and helps ensure timely access to therapies.
Our new study, Clinical and genetic characterisation of a large Indian congenital myasthenic syndrome cohort is now available in BRAIN: a journal of neurology online ahead of print. The publication is authored by CHEO’s postdoctoral researcher and CIHR fellowship recipient Kiran Polavarapu together with Sunitha Balaraju, with Hanns Lochmüller and Atchayaram Nalini as senior authors.
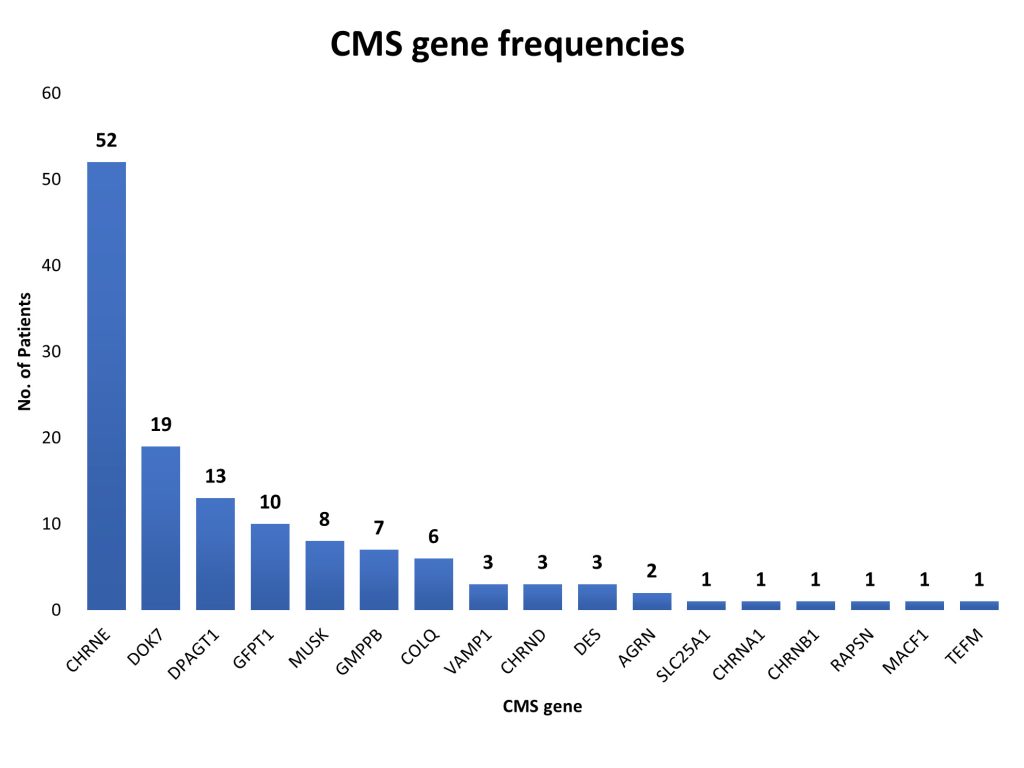
In this important collaboration with colleagues at the National Institute of Mental Health and Neurosciences in Bangalore under the leadership of Dr Nalini, we characterised 156 genetically diagnosed patients from 141 families, describing the mutational spectrum and genotype-phenotype correlation. Disease-causing variants in 17 CMS-associated genes were identified in 93.6% of families involved in the study, while in 6.4% of families, variants in genes not typically associated with CMS were found. This study highlights the geographic differences in the frequencies of various causative CMS genes and underlines the increasing significance of glycosylation genes (DPAGT1, GFPT1 and GMPPB) as a cause of neuromuscular junction defects. The novel genes MACF1 and TEFM identified in this cohort add to the expanding list of genes with new mechanisms causing neuromuscular junction defects.
This study used the RD-Connect genome phenome analysis platform (GPAP) for identification of causative variants in exome datasets from many of the cases involved. Dr Kiran Polavarapu and the Lochmüller lab team including co-author Dr Rachel Thompson make extensive use of the GPAP to analyse and solve difficult to diagnose cases of CMS and to find second families that help confirm novel gene discoveries.
How to Find the Publication
The paper is available on the Brain website here. If you do not have access through your institution, please contact Dr Hanns Lochmüller to obtain a full-text version.
Reference
Polavarapu K, Sunitha B, Töpf A, Preethish-Kumar V, Thompson R, Vengalil S, Nashi S, Bardhan M, Sanka SB, Huddar A, Unnikrishnan G, Arunachal G, Girija MS, Porter A, Azuma Y, Lorenzoni PJ, Baskar D, Anjanappa RM, Keertipriya M, Padmanabh H, Harikrishna GV, Laurie S, Matalonga L, Horvath R, Nalini A, Lochmüller H. Clinical and genetic characterisation of a large Indian congenital myasthenic syndrome cohort. Brain. 2023 Sep 18:awad315. doi: 10.1093/brain/awad315. Epub ahead of print. PMID: 37721175.
Abstract
Congenital myasthenic syndromes are a rare group of inherited disorders caused by gene defects associated with the neuromuscular junction and potentially treatable with commonly available medications such as acetylcholinesterase inhibitors and beta2 adrenergic receptor agonists. In this study we identify and genetically characterise the largest cohort of congenital myasthenic syndrome patients from India to date. Clinically suspected patients evaluated in a South Indian hospital between 2014-2019 underwent genetic testing either by standard diagnostic methods of gene panel testing or a two-step method of hotspot screening followed by whole-exome sequencing. In total 156 genetically diagnosed patients (141 families) have been characterised in this study and the mutational spectrum and genotype-phenotype correlation described. 87 males and 69 females were evaluated with an age of onset ranging from congenital to fourth decade (mean 6.6 ± 9.8 years). The mean age at diagnosis was 19 ± 12.8 (1-56 years) with a mean diagnostic delay of 12.5 ± 9.9 (0-49 years). Disease-causing variants in 17 congenital myasthenic syndrome-associated genes were identified in 132 families (93.6%), while in nine families (6.4%) variants in genes not associated with congenital myasthenic syndromes were found. Overall, postsynaptic defects were most common (62.4%) followed by glycosylation defects (21.3%), synaptic basal lamina genes (4.3%) and presynaptic defects (2.8%). Other genes found in our cohort to cause neuromuscular junction defects (DES, TEFM) accounted for 2.8%. Among the individual congenital myasthenic syndrome genes, the most commonly affected gene was CHRNE (39.4%) followed by DOK7 (14.4%), DPAGT1 (9.8%), GFPT1 (7.6%), MUSK (6.1%), GMPPB (5.3%) and COLQ (4.5%). We identified 22 recurrent variants in this study, out of which eight were found to be geographically specific to the Indian subcontinent. Apart from the known common CHRNE variants p.E443Kfs*64 (11.4%) and DOK7 p.A378Sfs*30 (9.3%), we identified seven novel recurrent variants specific to this cohort including DPAGT1 p.T380I and DES c.1023 + 5G > A for which founder haplotypes are suspected. This study highlights the geographic differences in the frequencies of various causative congenital myasthenic syndrome genes and underlines the increasing significance of glycosylation genes (DPAGT1, GFPT1 and GMPPB) as a cause of neuromuscular junction defects. Myopathy and muscular dystrophy genes like GMPPB and DES presenting as gradually progressive limb girdle congenital myasthenic syndromes expand the phenotypic spectrum. The novel genes MACF1 and TEFM identified in this cohort add to the expanding list of genes with new mechanisms causing neuromuscular junction defects.